9+ Which Intervals Are Affected By The Addition Of A Catalyst
A catalyst lowers the activation energy for example by providing a surface for the reaction. Reactions can be sped up by the addition of a catalyst including reversible reactions involving a final equilibrium state.
A Conserved Mechanism For Sulfonucleotide Reduction Plos Biology
Chemistry questions and answers.

. How are the following aspects of a reaction affected by the addition of a. Web Catalysts speed up chemical reactions. 1 1 and 2 2 1 and 3 3 2 and 4 4 3 and 4.
Reaction Coordinate Which intervals are affected by the addition of a catalyst. The reaction that produces ammonia is represented by balanced. Web Which statement describes the energy of the particles in this sample during interval DE.
A I and 2 C 2 and4 B. 7 _ÿcŒOšzS 3 1 and 3 4 3 and 4 1 1 and 2 2 2 and 5. How are the following aspects of a reaction affected by the addition of a catalyst.
Web Although catalysts have no effect on the position of equilibriumiethe yield of the reaction they do allow equilibrium to be reached more quickly. Web Adding a catalyst will decrease the activation energy both in the forward direction and the backward direction. Web Which intervals are affected by the addition of a catalyst.
Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of the reaction. Web Which intervals are affected by the addition of a catalyst. AH2O BNO2 CC2H6 DCO2 5According to Reference Table I which gas is formed from its elements as a.
Increased Decreased Not affected rate. Web The Effect of a Catalyst on Equilibrium. Web a catalyst was added to trial 2 Copper II ions Cu s I2 g - CuI2 s the reaction represented in the graph is best described by which of the following terms.
In summary -catalysts increase. 1 Both potential energy and average kinetic energy increase. Web May 26 2014 Rate of reaction can be increased by using a catalyst.
Web Which intervals are affected by the addition of a catalyst. Web Which intervals are affected by the addition of a catalyst. Web Given the potential energy diagram for a reaction.
Given the potential energy diagram for a chemical. When this happens the rate of the reaction will. Web How are the following aspects of a reaction affected by the addition of a catalyst.
Web A catalyst is a compound or element that increases the rate of a chemical reaction eg. This is really because. Aa catalyst Ban indicator Celectrical energy Dthermal energy 5The activation energy of a chemical reaction can.
The speed at which it occurs without itself being part of the reaction.

Effective Factors On Performance Of Zeolite Based Metal Catalysts In Light Hydrocarbon Aromatization
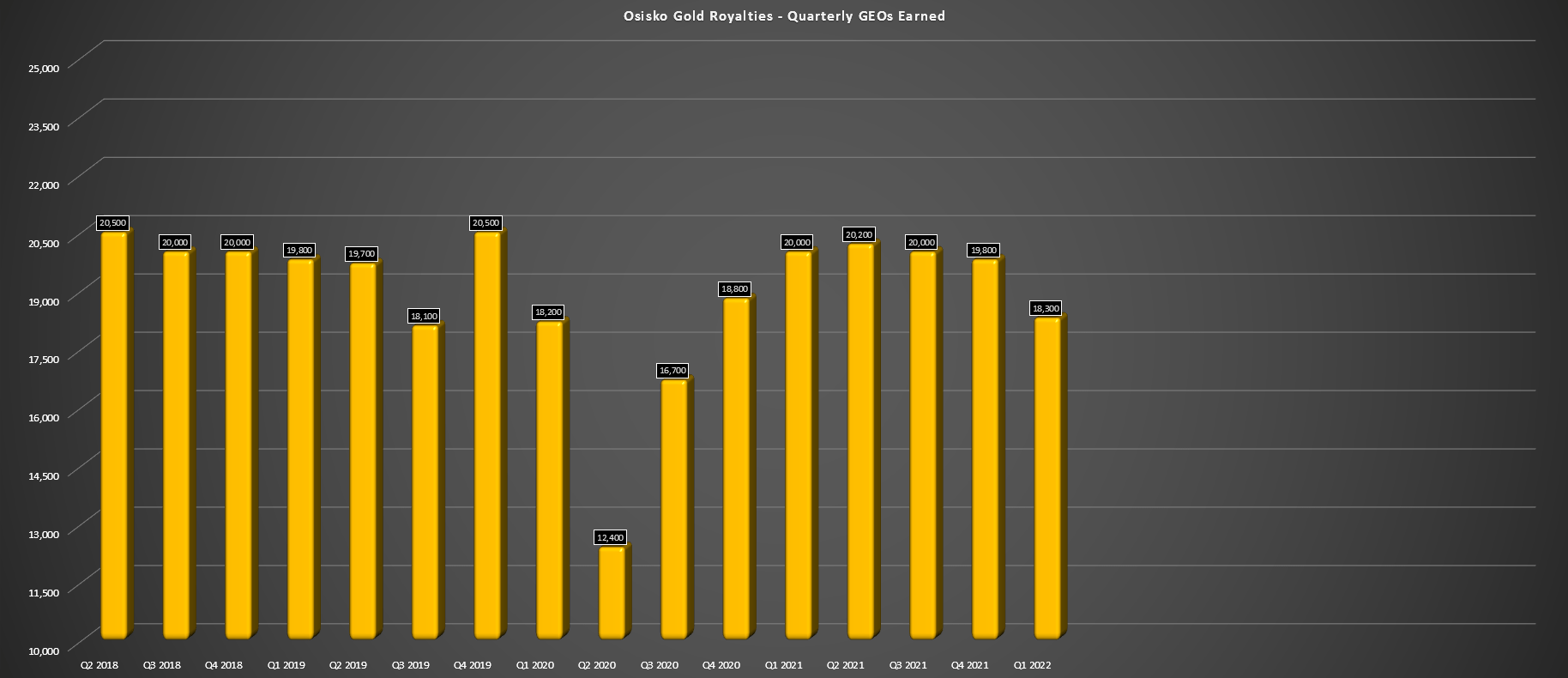
Osisko Gold Royalties Stock Buy The Dips Nyse Or Seeking Alpha

Chem 12
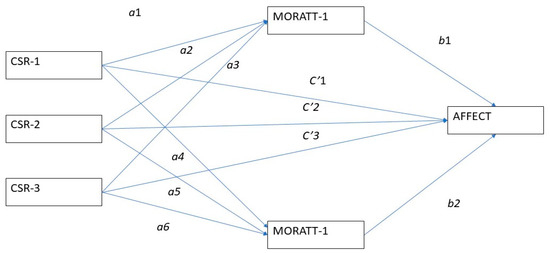
Sustainability Free Full Text Could Csr Practices Increase Employee Affective Commitment Via Moral Attentiveness Html

Given The Potential Energy Diagram For A Reaction Which Intervals Are Affected By The Addition Of Brainly Com
List References From The University Of Geneva Physical Chemistry Reference Database

App Store Connect Help

Chemistry Kinetics And Equilibrium Flashcards Quizlet

Ex 99 1

425

Chemistry Kinetics And Equilibrium Flashcards Quizlet
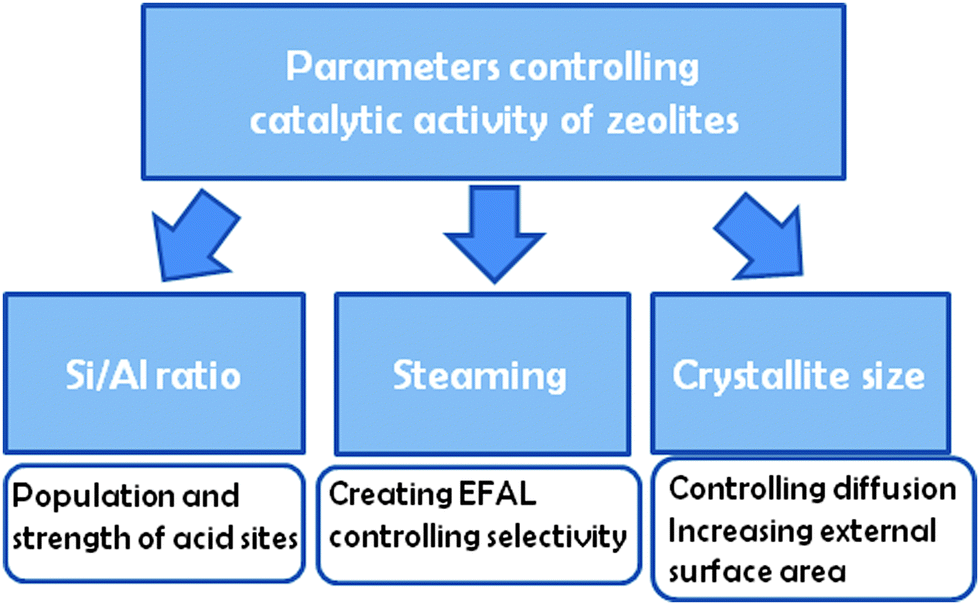
Zeolites As Catalysts In Oil Refining Chemical Society Reviews Rsc Publishing Doi 10 1039 C3cs60394f
Regents Chemistry Exam Explanations January 2016

Single Pot Catalyst Strategy To Branched Products Via Adhesive Isomerization And Hydrocracking Of Polyethylene Over Platinum Tungstated Zirconia Sciencedirect
Regents Chemistry Exam Explanations January 2013
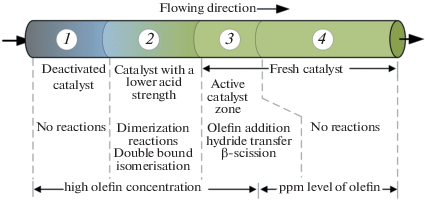
Comparison Of Isobutane N Butenes Alkylation Over Y Zeolite Catalyst In Cstr Fixed Bed And Circulating Flow Reactors Springerlink
All Abstracts Apcche2019